Our Policy Recommendations
- Home >
- Our Policy Recommendations
AMR Alliance Japan Policy Recommendations: The Japanese Government’s Role in Promoting AMR Countermeasures
Every year, approximately 700,000 people are estimated to die from antimicrobial resistance (AMR) related causes globally. Without action on this issue, it is projected that this figure will rise to up to 10 million people per year by 2050.*1
AMR is a growing problem. In Japan, cases of antimicrobial resistant infections are on the rise both inside and outside of medical institutions, especially related to Methicillin-resistant Staphylococcus aureus (MRSA), penicillin-resistant Streptococcus pneumonia (PRSP), Extended-spectrum beta-lactamases (ESBLs), multi-drug resistant Pseudomonas aeruginosa (MDRP), and carbapenem-resistant Enterobacteriaceae (CRE).*2 In recent years, Japan has also seen the rise of drug-resistant fungal infections.*3
In light of the above, and based on the situation in Japan surrounding AMR right now, three years after the establishment of the Japanese Government’s National Action Plan on Antimicrobial Resistance, AMR Alliance Japan*4, a multi-stakeholder organization advocating for stronger AMR policies, proposes the following recommendations on AMR.
These recommendations cover the six themes of “Promoting antimicrobial stewardship based on the actual situation at healthcare facilities,” “Constructing domestic AMR surveillance and crisis management systems,” “Enabling the further and active use of AMR screening methods and rapid diagnostic technology,” “Supporting education on AMR for the public and medical practitioners,” “Establishing incentive models to encourage R&D for antimicrobials,” “Stabilizing the supply of antimicrobials,” and “International collaborations to share case studies and lessons learned about AMR.”
AMR is a complex issue that requires a multifaceted response. Thus, it is believed that each of the Alliance’s policy recommendations will be strengthened and supported by the achievement of the other recommendations. The relationship between each policy recommendation theme is detailed in a chart on the next page.
- *1 O’Neill, J. “Tackling Drug-Resistant Infections Globally: Final Report and Recommendations”. Review on Antimicrobial Resistance. May 2016.
- *2 “Nihon no Yakuzaitaiseikin no Jyokyo (The AMR situation in Japan)”. National Center for Global Health and Medicine, Antimicrobial Resistance Clinical Reference Center. http://amr.ncgm.go.jp/general/1-3-1.html
- *3 “Candida auris.” Centers for Disease Control and Prevention, National Center for Emerging and Zoonotic Infectious Diseases (NCEZID), Division of Foodborne, Waterborne, and Environmental Diseases (DFWED), https://www.cdc.gov/fungal/candida-auris/index.html (accessed: May 21, 2019)
- *4 Established in November 2018 with the Health and Global Policy Institute as the Alliance Secretariat. Current members as of June 2019 are, in alphabetical order, The Japan Pharmaceutical Manufacturers Association, The Japanese Association for Infectious Diseases, The Japanese Society for Clinical Microbiology, The Japanese Society for Infection Prevention and Control, The Japanese Society for Medical Mycology, The Japanese Society for Pediatric Infectious Diseases, The Japanese Society of Chemotherapy, The Japanese Society of Pharmaceutical Healthcare and Sciences, The Japanese Society of Therapeutic Drug Monitoring, The Pharmaceutical Society of Japan, MSD K.K., Nippon Becton, Dickinson and Company, Ltd., and Shionogi & Co., Ltd.
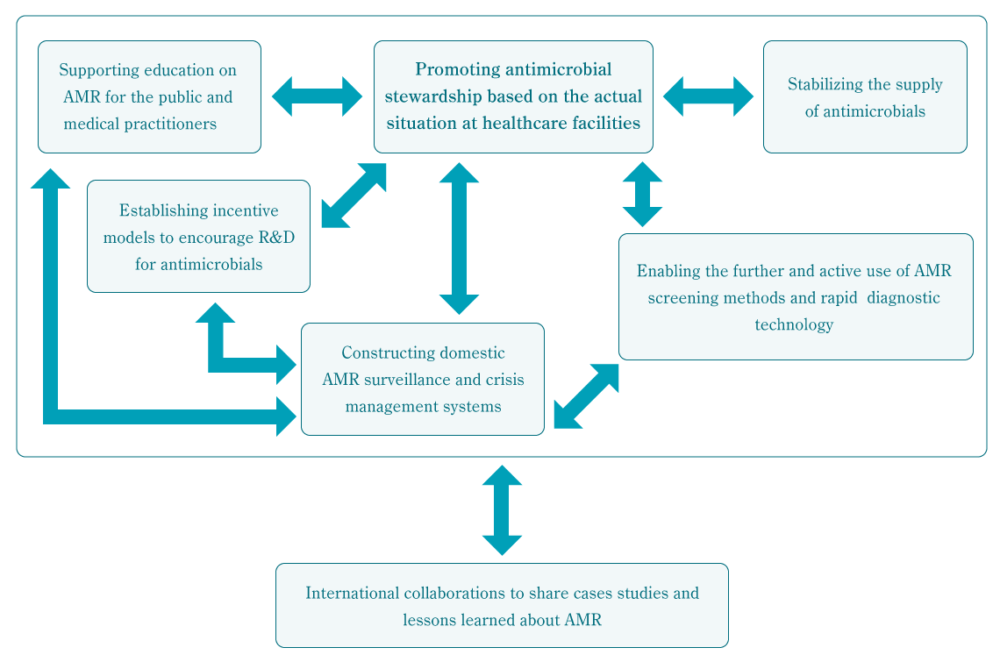
“Promoting antimicrobial stewardship based on the actual situation at healthcare facilities” is tied to all of the other themes. The construction of domestic AMR surveillance systems will contribute to the information base needed by the themes of “Supporting education on AMR for the public and medical practitioners” and “Establishing incentive models to encourage the R&D for antimicrobials.” Surveillance itself is supported by recommendations within the theme of “Enabling the further and active use of screening methods for the detection of antimicrobial-resistance and rapid diagnostic technology.” Given the threat that AMR problems overseas pose for Japan, “International collaborations to share cases studies and lessons learned about AMR” is seen as a crucial theme supporting all of the other recommendations.
- 01
-
Promoting antimicrobial stewardship based on the actual situation at healthcare facilities
There is currently ongoing debate on whether the numeric targets used within the National Action Plan on Antimicrobial Resistance released in April 2016 might be reducing the use of antimicrobials excessively, even in cases where antimicrobial use is actually warranted.
There is also a need to further promote the use of the Manual of Antimicrobial Stewardship developed by the Ministry of Health, Labour and Welfare.
- Policies discouraging the inappropriate use of antimicrobials are crucial to the promotion of antimicrobial stewardship. However, the Government should consider on how to promote antimicrobial stewardship in such a way that does not discourage appropriate use.
- AMR Alliance Japan proposes the following measures intended to encourage the use of the Manual of Antimicrobial Stewardship in medical facilities among non-infectious disease specialists:
- Add text explaining when antimicrobials can be used for common illnesses
- Add lists of effective antimicrobials by patient condition
- AMR Alliance Japan requests that the Japanese Government consider revising the medical fee system to fund the following measures encouraging antimicrobial stewardship.
- The establishment of infectious disease departments in all university hospitals and central hospitals, and the dispatch of specialists to handle infectious disease treatment (including the treatment of hospital-acquired infectious diseases, and support for infectious disease treatment in smaller communities). This should be accompanied by the creation of a system to dispatch doctors and pharmacists to both Antimicrobial Stewardship Teams (ASTs) and Infection Control Teams (ICTs) in university and central hospitals thereby enabling coordination between the teams.
- The establishment of ASTs in every medical facility across the country, and the creation of a system that ensures that the efforts of specialists in these teams are properly rewarded.
- Guidance for medical facility management to ensure that profits earned from the medical fee system are used to sustain and further improve the quality of infectious disease treatment and ASTs.
- AMR Alliance Japan requests that text be added to the language on specific uses for pharmaceuticals currently being considered by the Government for inclusion in the revision of the Act on Securing Quality, Efficacy and Safety of Products Including Pharmaceuticals and Medical Devices in order to make allowances for changes in the way that antimicrobials are used out of a consideration for AMR.
- 02
-
Constructing domestic AMR surveillance and crisis management systems
There is no centralized authority overseeing nationwide AMR surveillance in Japan in such a way to enable effective crisis management measures. It is not possible to follow patients after they receive antimicrobials, and current surveillance data is not linked to clinical data or data on susceptibility.
- The Government should recognize AMR as a global-scale threat to humanity and take responsibility for the creation of a sustainable and comprehensive surveillance system operated through public-private cooperation. The Government should invest appropriately in that system based on that recognition.
- In constructing such a system, the Government should take into consideration the following points.
- The system should be able to capture detailed and real-time info on AMR and antimicrobial usage (including strain data and measurements of antimicrobial usage).
- The system should make use of a format that makes the effective transfer and collection of data easy, based on the opinions of clinical facilities, and a consideration of the burden the system might place on them.
- Data from facilities not currently implementing surveillance measures is of vital importance. A system should also be constructed to provide support to those facilities so that they can hire the personnel needed for surveillance work, which is of particular importance for the surveillance of outpatient facilities.
- 03
-
Enabling the further and active use of AMR screening methods and rapid diagnostic technology
AMR screening tests at the point of entry into the healthcare system are crucial for the prevention of the spread of AMR. Current testing technology can shorten the time required for the microorganism identification and susceptibility tests needed for rapid diagnoses to two days or less. However, not enough is being done to support efforts to secure the personnel needed to run such tests, or to enable medical facilities to invest in such technology.
- The Government should consider revising the medical fee system to fund the following AMR countermeasures.
- The spread of AMR screening tests and rapid diagnostic technology, which allow for accurate judgements about AMR, enabling the effective prescription or halting of antimicrobials.
- The promotion of training initiatives that can help medical facilities find the personnel to operate AMR screening tests, microorganism identification tests, and susceptibility tests (including genetic screening tests).
- Measures to appropriately cover the costs of tests that are currently borne by individual medical facilities (e.g. tests to identify the presence of ESBLs or CREs).
In revising the medical fee system, the Government should clarify regulations related to the dispatch of specialized staff and the installation of testing devices in order to resolve problems related to the lack of laboratory personnel and testing technology at medical facilities. Furthermore, the Government should make the submission of test data mandatory for medical facilities selected as important to the domestic AMR surveillance system.
- 04
-
Supporting education on AMR for the public and medical practitioners
Japan has achieved relatively good control over the spread of AMR compared to other countries. As a result, the public tends not to think of AMR as a serious issue. There is evidence that the misconception is spreading among the public that antimicrobials are effective against viral diseases.*5
- *5 Koukinyaku Ishiki Chosa 2018 (Antimicrobial Awareness Research 2018).” National Center for Global Health and Medicine, Antimicrobial Resistance Clinical Reference Center. http://amr.ncgm.go.jp/pdf/ig-8.pdf (accessed: May 21, 2019)
- The Government should work to increase the opportunities that the general public has to learn about AMR. The Government should consider how to appropriately support informational initiatives tailored to different audiences, such as the elderly or the young, through a variety of media sources, including newspapers, magazines, television, social media, and the internet. The Government should construct a cooperative system among school doctors, school pharmacists, and school nurses at educational and childcare institutions, and work to increase opportunities to accurately and actively promote information on infectious disease prevention, including information related to AMR and vaccines.
- The Government should increase the opportunities the public has to learn about the spread of infectious diseases and how they can be prevented by vaccines, disinfection practices, and so on.
- The Government should promote the further training of medical practitioners with specialized knowledge in infectious diseases (licensed specialists) that can contribute to AMR countermeasures.
- Growth in the number of medical personnel trained in therapeutic drug monitoring (TDM) and the expansion of the use of TDM for antimicrobials would help to further facilitate decisions about the appropriateness of antimicrobial use and hence, AMR countermeasures. The Government should consider supporting training and the expansion of TDM practices to more antimicrobials through revisions to the medical fee system.
- The Government should make use of the Conference on Public Awareness-raising for AMR Countermeasures and other fora to promote multi-stakeholder collaborations among medical personnel, patient advocates, insurance payers, industry, the Government, and experts on AMR, in order to reinforce current AMR educational activities. The Government should promote the creation of opportunities for community doctor associations, pharmacist associations, health centers and so on to collaborate for the promotion of awareness-raising in their communities.
- 05
-
Establishing incentive models to encourage R&D for antimicrobials
It is difficult to predict the profitability of new antimicrobials. Companies are faced with a situation in which they may spend ten years or longer developing a new antimicrobial, only to have its use actively discouraged upon release. Incentives are needed to help companies move R&D forward.
- Pull incentives should be implemented to encourage the development of antimicrobials. Specifically, market entry rewards, transferable exclusivity extensions, purchase guarantee systems, and pre-examination pricing systems based on drug profiles should be considered.
- Japan should consider the introduction of new domestic financing mechanisms to promote the R&D of novel antimicrobials, and thereby assume global leadership on antimicrobial development.
- 06
-
Stabilizing the supply of antimicrobials
Problems ensuring the stable supply of antimicrobials can lead to situations such as the delay or halting of infectious disease treatments, or the use of non-recommended antimicrobials for treatments, which may further spread AMR. Conversely, pharmaceutical companies consistently face a hard time in acquiring raw materials for antimicrobials and difficulties due to imbalances in the production costs and set selling prices for antimicrobials (for instance, Cefazolin, Panipenem/Betamipron, and Ampicillin/Sulbactam). Threats exist to the stable supply of alternative antimicrobials as well.
- The Government should invest appropriately to help companies realize the stable supply of antimicrobials, based on a risk-management perspective (including through the consideration of increasing the premiums attached to antimicrobials within the medical fee system).
- The Government should support companies in their efforts to ensure that multiple production routes exist for regularly used antimicrobials, and to make it possible to increase the supply of antimicrobials when needed.
- 07
-
International collaborations to share case studies and lessons learned about AMR
Developing countries face many problems related to AMR, including a lack of transparency around data on the occurrence of AMR and the usage of antimicrobials, and issues related to counterfeit antimicrobials. AMR problems can cross national borders at any time. International cooperation is necessary in order to truly prevent the spread of AMR.
- Efforts for the Asia-Pacific One Health Initiative on AMR (ASPIRE) should be commended. Japan should continue to request cooperation and call on other counties based on a One Health approach in order to prevent the spread of AMR.
- The Government should construct a domestic surveillance system linking data on measures to promote antimicrobial stewardship, information on the incidence of AMR infections, and information on the use of antimicrobials. Having done so, the Government should spread that system to Asia and the rest of the world, thereby assuming global leadership on AMR countermeasures.
- The Government should enhance support for initiatives to provide guidance on the use of antimicrobials in developing countries, based on the recognition that active support for countries overseas will lead to the strengthening of domestic AMR countermeasures. In undertaking support for developing economies, the Government should promote the creation of a cooperative system that will dispatch not only licensed medical professionals, but other personnel with needed skills and knowledge as well.


